Similar to humans, the feet of pigs bear the weight and undergo considerable strain with little care. It’s crucial to prioritise foot health in pigs just as we do for ourselves. Many of us might not regularly check our pigs’ feet or understand what signs to look for.
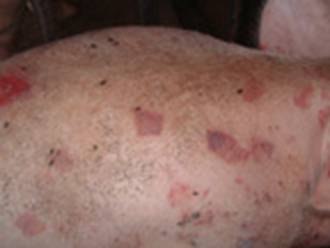
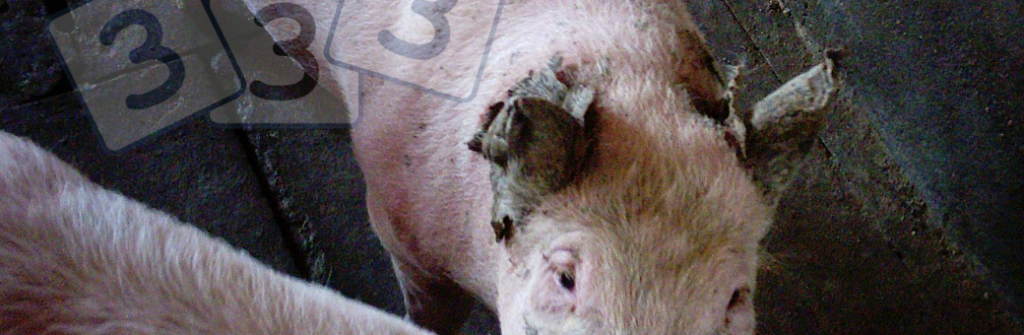
Damage to the Oxford Sandy and Black pig feet leading to infection is a common cause of lameness and welfare issues among pigs, as observed by some sharing photos with us over the months on the Oxford Sandy and Black Pig Group on facebook . This problem affects all age groups, with sows and boars posing a particular challenge due to increased weight bearing, longevity, and variable nutritional status throughout their lives. The genetic makeup of the Oxford Sandy and Black pig can also contribute to these issues.
The majority of foot problems stem from defects in the claw, resulting from abnormal and excessive growth, abrasion, or a combination of both. Secondary contamination often leads to abscess formation and septic laminitis. Larger outer claws, bearing the most weight, are especially vulnerable to damage, with conditions like bush foot notably affecting the outer claws of the hind limbs.

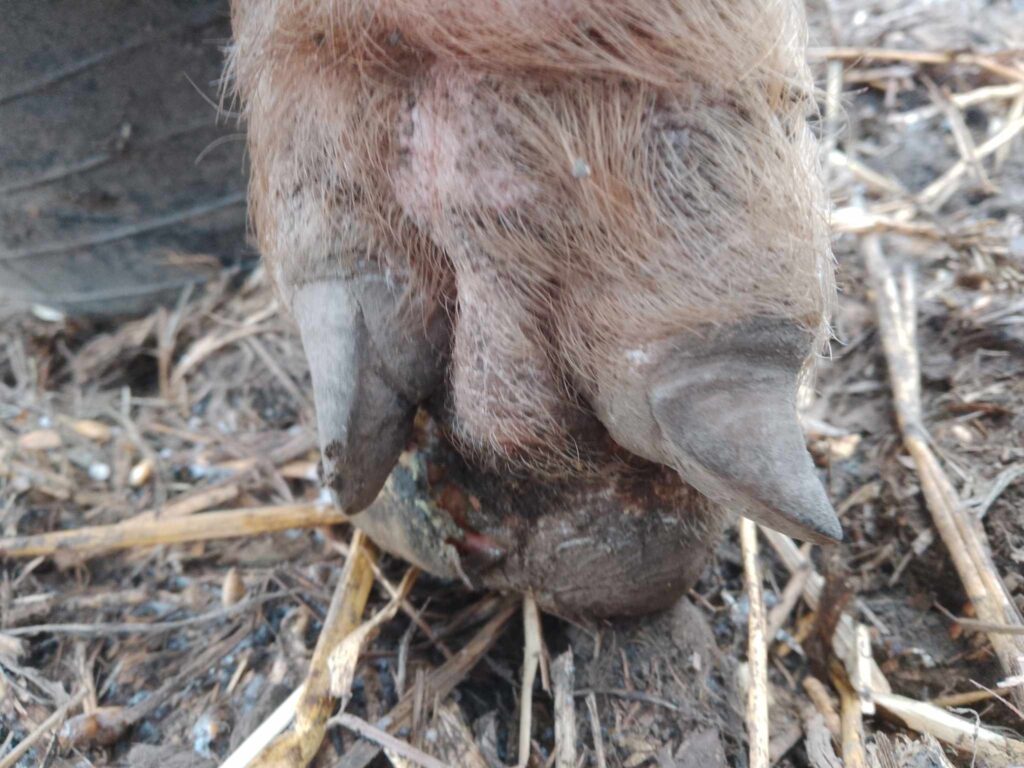
Prevention is key, and maintaining hoof integrity should be a primary focus for all of us to ensure the well-being of our pigs.
Horn Growth and Foot Care in Pigs
The continuous growth of the protein-based (keratin) tissue in pig claws requires an appropriate protein level in their diet. Excessive protein can stimulate deformities and overgrowth, as discussed in previous posts. Sow protein requirements fluctuate through the reproductive cycle, impacting horn growth. Micronutrients like biotin, available at farm supply stores, are crucial for horn development.
Environmental conditions play a role in horn growth and hoof integrity. Wet ground, abrasive surfaces, and chemical exposure can affect pig hooves. Wet concrete poses a risk, as it softens hooves, potentially leading to overgrowth and cracks.
Consequences of poor hoof health include difficulty rising, altered gait, and susceptibility to infections like bush foot. Bacteria such as Streptococci, Staphylococci, E. coli, Fusiformis, and Trueperella pyogenes can cause infections through defects in the horn.
Foot care aims to maintain healthy, well-shaped hooves. We should aim and ensure that our pregnant and non-productive adult pigs receive at least 15% protein-based ration. Grazing variations and grass intake contribute to nutritional and a good enhancement on well being to our pigs.
For overgrown hooves, clipping and filing can be done, and sedation is also an option. Copper sulphate hoof mats, used weekly, can address feet problems. Other foot dip treatments should be carefully chosen, as some may harden hoof horn, leading to brittleness. Disposal of chemical solutions must be done with utmost care to prevent environmental contamination.
Remember, a healthy pig is a happy pig and a very happy keeper:-)
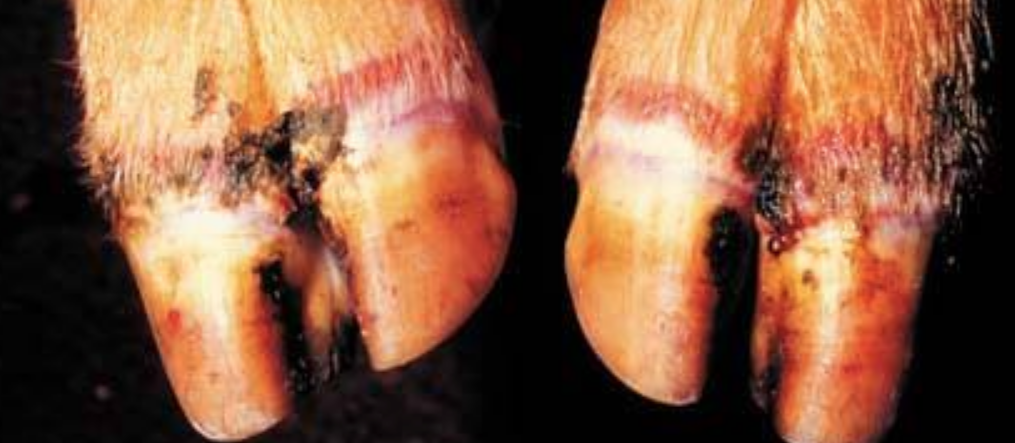
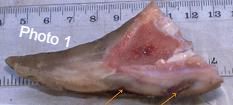
Photo 1 showing: Overgrown claw highlighting defects whereby infection can harbour
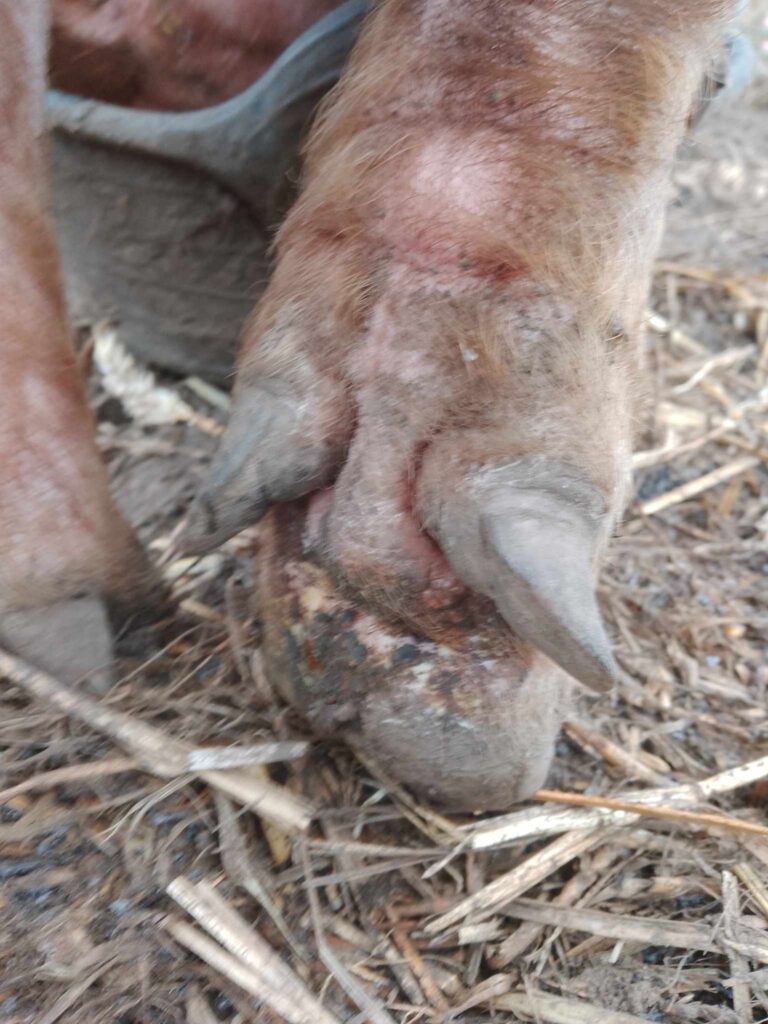
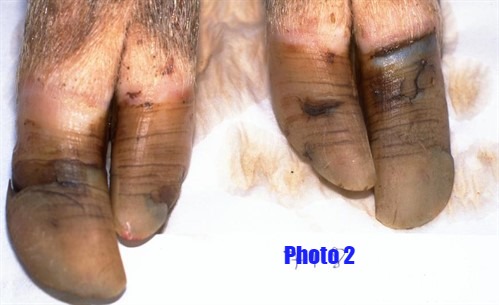
Photo 2 showing: Overgrowth of claws which may make rising difficult and will alter the gait of the pig which can put additional strain on joints
PHOTOS: PURDUE UNIVERSITY
The Oxford Sandy and Black Pig Group is UK’s only pig breed that is a registered charity in England & Wales (1190469) and Scotland (SCO52662). We are creating a better future for our breed the bloodlines and it breeding potential together with our Independent Pork Producers, Breeders and Oxford Sandy and Black Keepers. Please click the donate button so we may continue to look after our breed and our supporters
Follow us on Facebook and see how we support, help and inspire individuals about our rare breed